Water Quality and Herbicide Efficacy
Applying herbicides, insecticides, fungicides, or foliar fertilizers either tank-mixed or applied in separate applications is common practice in agriculture. Water is the primary vehicle in which pesticides are dissolved and tank-mixed with other components. Depending on the quality and quantity of the water and spray solution, pesticide efficacy may be compromised. Water quality refers to the chemical, physical, and biological characteristics of water utilized for these applications. A variety of factors, including water pH, minerals dissolved, hardness, and turbidity can determine how pesticides will perform when applied to their intended targets.
Pesticides purchased for agricultural purposes are composed of active and inert ingredients. Some of the active ingredients may not mix well with water alone, so inert ingredients are included in the formulation to promote solubility of active ingredients. Inert ingredients may also be added to ease application, extend product shelf life, improve safety for the applicator, and/or protect the pesticide from degradation. Adjuvants are also commonly included in the pesticide formulation to improve mixing and application or to enhance pesticide activity.
Acidity or Basicity (pH)
In chemistry, pH represents the acidity and basicity of a solution. The pH scale indicates hydrogen ion concentration in water, ranging from 0 to 14, with 7 being neutral. A higher H+ concentration lowers the pH below 7 and acidity increases. Basic solutions have pH values higher than 7. A pH change of one unit, (for example, 8 to 7) corresponds to a 10x change in H+ concentration in a solution.
At room temperature, pure water has a pH value of 7. Rain typically has a pH of 5.6 when it is equilibrated with atmospheric carbon dioxide. Soil water in contact with decaying organic material can have a pH of 4. Based on U.S. Geological Service data, groundwater pH value typically ranges from 6.0 to 8.5, depending on the type of soil and geological substrate.
Knowing the pH value of water used for pesticide application is particularly important since certain pesticides undergo a chemical reaction in the presence of alkaline or acidic solutions. In this process, called hydrolysis, the effectiveness of an active ingredient may be reduced. The rate at which the pesticide breaks down depends on its chemical properties, the pH of the mix water, and the amount of time the pesticide is in solution. For weak acid herbicides such as 2,4-D and glyphosate, when the pH value of mixing water is between 8 and 9, rapid hydrolysis occurs, breaking down the pesticide and possibly reducing pest control. The same occurs with weak alkaline herbicides such as metsulfuron-methyl when mixed in acid solution (pH less than 7). Half-life is the time it takes for one-half of the amount of pesticide in the water to degrade (50 percent hydrolysis). As shown in Table 1, different active ingredients will have different half-lives based on pH level of the mixture.
Due to chemical breakdown at different solution pH, pesticide manufacturers often add pH buffers (compounds that prevent sudden pH changes) to herbicide formulations that reduce the influence of water pH on the final pH of the solution. For example, when mixing glyphosate or glufosinate in solution, because of the herbicide formulation and high rate (more than 22 fluid ounces per acre), the final solution pH will be closer to the ideal pH of those herbicides. Figure 2 illustrates the comparison of solution pH before (6.60) and after glyphosate (4.48) or glufosinate (7.18).
Conversely, the initial pH of the solution will enhance some chemical properties of herbicides. Dicamba, for example, becomes more volatile (turns into vapor easily) when mixed in a solution with lower pH (less than 5). This will increase the potential for off-target movement from volatility. Due to this possibility, we recommend the use of adjuvants called volatilization reducing agents (VRAs), which are products that can help to keep spray solution pH within an ideal range for this herbicide.
Hardness
There are dissolved minerals such as calcium, magnesium, and iron in every source of water in nature. Unlike suspended solids, minerals do not settle, so their concentration is an important determining factor of water quality. Water hardness is a measure of calcium and magnesium concentration in water; the higher the concentration, the harder the water. In contrast, as sodium and potassium ions dissolve, water becomes “soft.” Total hardness is measured in parts per million or in grains of calcium and magnesium per gallon of water. One grain (65 milligrams) is approximately 17.1 ppm. According to the World Health Organization, water classification based on hardness is as follows: soft (0 to 114 ppm), moderately hard (114 to 342 ppm), hard (342 to 800 ppm), and extremely hard (more than 800 ppm).
Water hardness can affect some pesticides negatively because herbicide molecules and minerals will interact like magnets. In other words, negatively charged herbicides are attracted to positively charged minerals such as aluminum, iron, magnesium, calcium, and sodium. Once minerals bind to an herbicide molecule, herbicide chemical properties change, making them easier to be precipitated and harder to be absorbed by plants. Chemically altered molecules may be incapable of dissolving in water, penetrating leaf tissue, attaching to the site of action inside the pest to disrupt its biological functions, or even acting as a pesticide in some instances. Some herbicide labels indicate that a water conditioner may increase the performance of the product on annual and perennial weeds, particularly under hard water conditions.
Table 1. The half-life of selected pesticides at different solution pH values.
|
Common name |
Trade name |
pH 5 |
pH 6 |
pH 7 |
pH 8 |
pH 9 |
|
Acephate |
Orthene® |
40 Days |
— |
46 Days |
— |
16 Days |
|
Carbaryl |
Sevin® |
— |
125 Days |
24 Days |
2.5 Days |
1 Day |
|
Diazinon |
Knox-Out® |
31 Days |
— |
185 Days |
— |
136 Days |
|
Dicamba |
Banvel® |
Stable |
Stable |
Unstable |
Unstable |
Unstable |
|
Dimethoate |
Cygon® |
— |
12 Hours |
— |
— |
48 Minutes |
|
Malathion |
Digon® |
— |
8 Days |
3 Days |
19 Hours |
— |
|
Paraquat |
Gramoxone® |
Stable |
Stable |
Stable |
Unstable |
Unstable |
|
Trifluralin |
Treflan® |
Stable |
Stable |
Stable |
Stable |
— |
|
2,4-D amine |
Weedar® 64 |
Stable |
Stable |
Unstable |
Unstable |
Unstable |
(Deer and Beard 2001; Mckie et al. 2002)
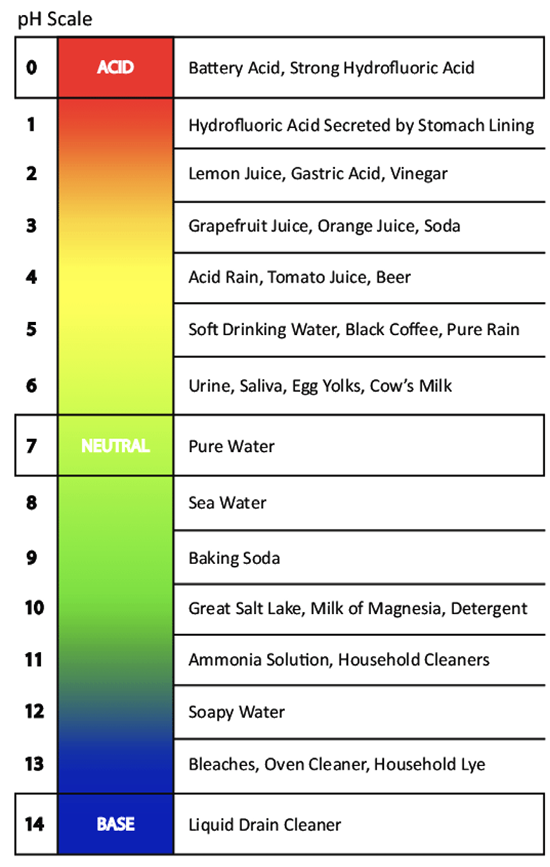
Figure 1. pH scale with common examples. Image adapted from Berg et al. 2007.



Figure 2. pH values for tap water (6.60), glyphosate solution (4.48), and glufosinate solution (7.18).
General Considerations
Water sources need to be tested for suitability when spraying pesticides. Correcting the pH or solving hardness problems depends on the specific pesticide label restrictions. To overcome the effects of water hardness when tank-mixing herbicides such as glyphosate, glufosinate, and 2,4-D, add ammonium sulfate fertilizer at a rate of 8.5 to 17 pounds per 100 gallons of water in spray solution first for hard to moderately hard water (less than 114 ppm).
References
Berg, M., M. McFarland, N. Dictson, J. Peterson, and D. Boellstorff. 2007. Texas Watershed Steward Handbook: A Water Resource Training Curriculum. AgriLIFE Extension, Texas A & M University.
Deer, H. M., and R. Beard. July 2001. Effect of water pH on the chemical stability of pesticides. Utah State University Fact Sheet. AG/Pesticides/14.
Whitford, F., D. Penner, B. Johnson, L. Bledsoe, N. Wagoner, J. Garr, K. Wise, J. Oberneyer, and A. Blessing. 1986. The impact of water quality on pesticide performance. Purdue University Extension. PPP-86.
The information given here is for educational purposes only. References to commercial products, trade names, or suppliers are made with the understanding that no endorsement is implied and that no discrimination against other products or suppliers is intended.
Publication 3896 (POD-05-23)
Antonio Augusto Correa Tavares, Graduate Research Assistant, Department of Plant and Soil Sciences; Darrin Dodds, PhD, Professor and Head, Plant and Soil Sciences; and Cori Speights, PhD, Extension Associate I, Biochemistry, Molecular Biology, Entomology and Plant Pathology.
The Mississippi State University Extension Service is working to ensure all web content is accessible to all users. If you need assistance accessing any of our content, please email the webteam or call 662-325-2262.




